GTC has a new publication in Heliyon titled “The Potential of Cell-Free RNA in Liquid Biopsy: Comprehensive Analysis of Mutation Profile, Chromosomal Abnormalities, and Immune Biomarkers”. This study explores the potential of cell-free RNA (cfRNA) and AI as valuable components of liquid biopsy for evaluating the host immune system and predicting disease markers.
The study reveals key findings:
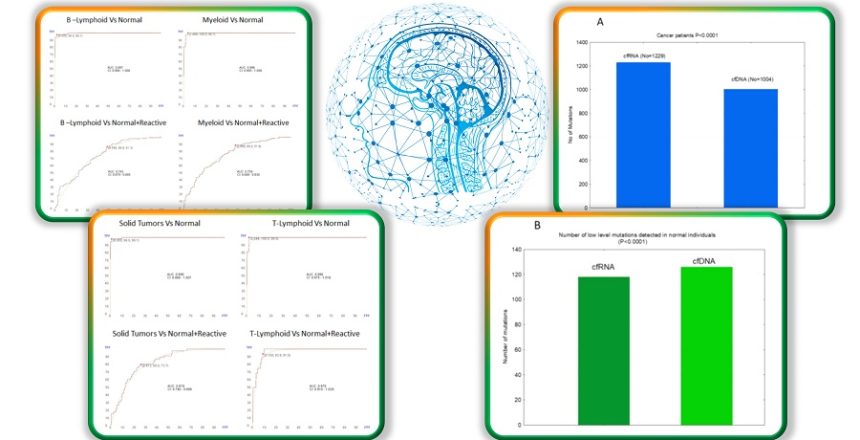
1. Mutation Profile and Chromosomal Abnormalities:
– Using a targeted RNA panel significantly more mutations were detected using cfRNA compared to cfDNA.
– cfRNA analysis provides comprehensive information on mutation profiling, chromosomal structural abnormalities, and fusion genes, increasing sensitivity and specificity for detecting minimal residual disease (MRD).
2. Immune Biomarkers and Host Response:
– cfRNA evaluation reflects the expression levels of important genes associated with specific cancers and the host immune response.
– cfRNA can provide information comparable to flow cytometry analysis of hematologic neoplasms and can evaluate immune system markers in various diseases.
3. Clinical Applications and Future Directions:
– Integrating cfRNA and cfDNA analysis enhances liquid biopsy capabilities.
– Machine learning algorithms can distinguish between normal and diseased states, predict therapy response, and develop specific diagnostic models.
– Larger studies are needed to confirm these findings and establish clinical applications for cfRNA-based liquid biopsy.
This study has significant implications for liquid biopsy research and clinical practice.