New publication on how cfRNA and AI are advancing LBx
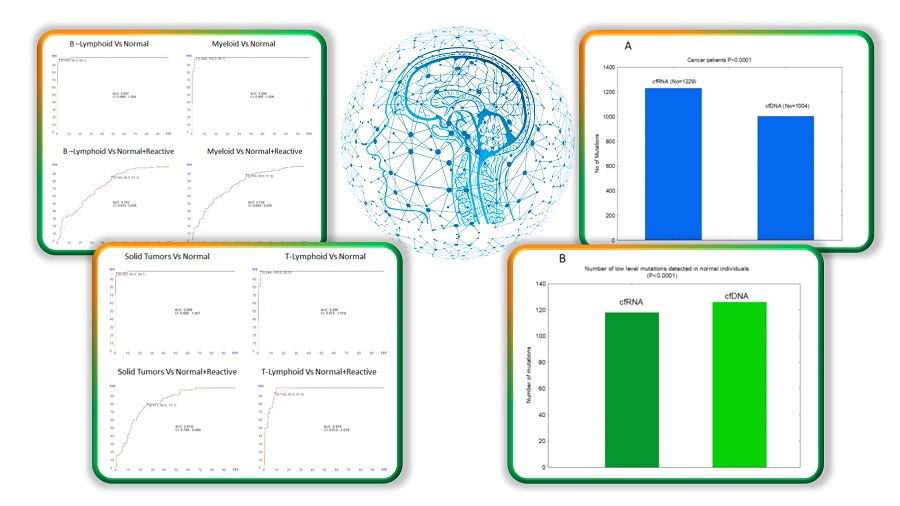
GTC has a new publication in Heliyon titled “The Potential of Cell-Free RNA in Liquid Biopsy: Comprehensive Analysis of Mutation Profile, Chromosomal Abnormalities, and Immune

GTC has a new publication in Heliyon titled “The Potential of Cell-Free RNA in Liquid Biopsy: Comprehensive Analysis of Mutation Profile, Chromosomal Abnormalities, and Immune

Baltimore, MD and Irvine, CA, April 13, 2023, Sysmex Inostics Inc., a subsidiary of Japan’s Sysmex Corporation and Baltimore-based biotechnology firm, and Genomic Testing Cooperative

Demonstrating Reliability of Targeted Transcriptomic Profiling in Predicting Levels of HER2, ER, PD-L1 and Other Immunohistochemistry-based Biomarkers when Combined with Artificial Intelligence Irvine, California– April

ASH is just over a month away. Come meet with the GTC team and its co-op partners at our happy hour event. Download your invitation
CHICAGO – With the publication of a paper validating two artificial intelligence-based algorithms for assisting pathologists in molecular profiling of patients, the Genomic Testing Cooperative

GTC research is being presented at ASCO 2020 in the following three abstracts. 1. FGFR expression, fusion and mutation as detected by NGS sequencing of

GTC featured in the Orange County Business Journal on September 30, 2019 [Click image to enlarge]

We’re always competing with ourselves on improving turn around time (TAT) because we know patients depend on the results Most studies suggest that treatment delay
MDS is defined as ineffective hematopoiesis characterized by adequate or increased hematologic elements in bone marrow with peripheral cytopenia. Therefore, the presence of cytopenia in
When you order GTC Breast Cancer Profile The FDA has approved Piqray (alpelisib) tablets, to be used in combination with the FDA-approved endocrine