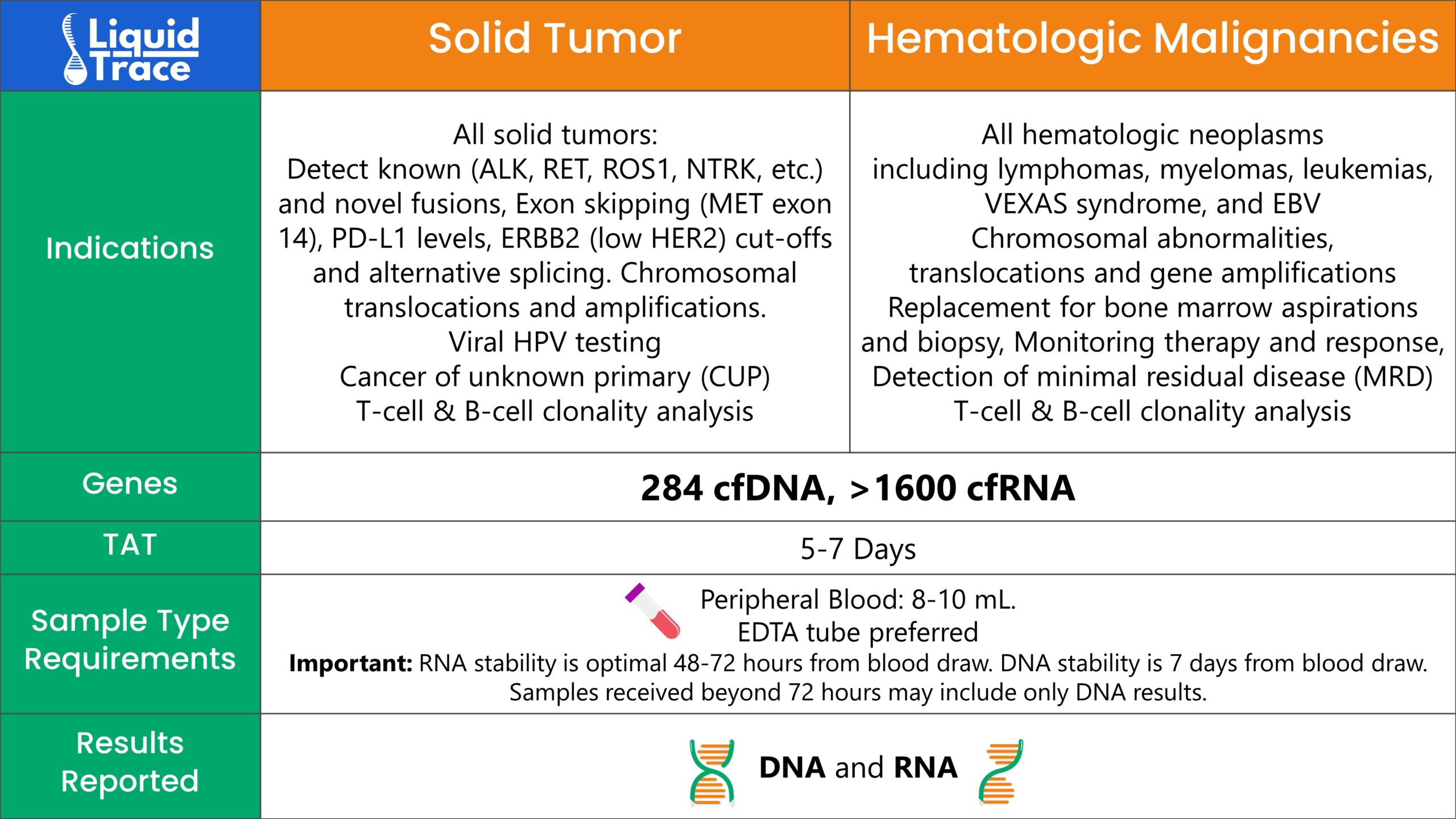
>302 cfDNA genes
>1600 cfRNA genes
Pan-Tumor Assay for Both Solid Tumors
and Hematologic Malignancies


GTC’s Liquid Trace can significantly reduce the need for bone marrow biopsies for hematology patients
Liquid biopsy in its current form is dependent on circulating cell-free DNA (cfDNA) analysis; this method likewise presents multiple challenges. These include variations in DNA shedding between tumors as well as low sensitivity (especially in early-stage cancer), difficulty in detecting fusion genes (i.e., chromosomal translocations leading to the expression of chimeric mRNA from two genes), and inability to reflect the numerous biological processes that modify RNA expression levels, such as alternative splicing, stability, and allele-specific methylation. The latter limitation is critically important as recent studies have shown that RNA testing provides another level of biological information regarding the tumor and its microenvironment.
GTC’s Liquid Trace is a pan-cancer test that evaluates cell-free RNA (cfRNA) and cfDNA and provides highly informative data that can be used for diagnoses, evaluating the host immune response, and identifying biomarkers for predicting responses to various therapies.
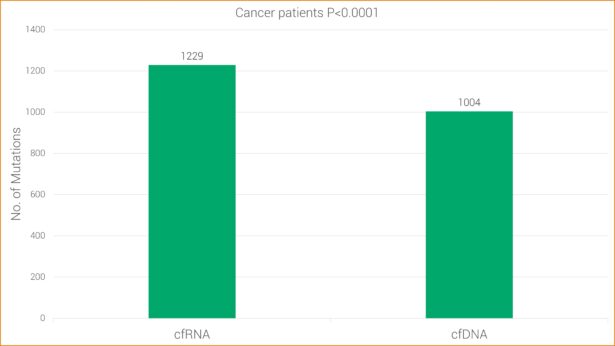
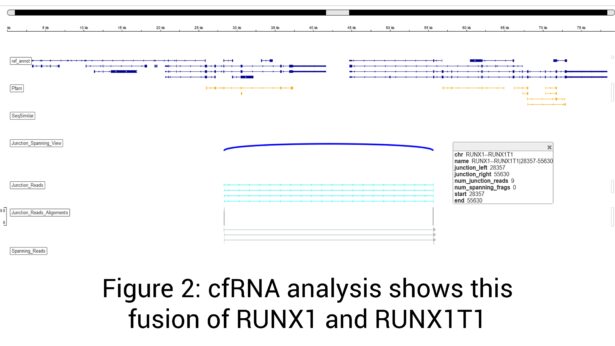
RNA sequencing has proven to be more sensitive in detecting mutations in clinical studies. This research is consistent with GTC’s findings that cfRNA has increased sensitivity over cfDNA alone. More specifically, cfRNA allows GTC’s Liquid Trace to detect mutations and fusions in hematologic and solid tumor samples that may be undetected with conventional cfDNA.
Liquid Trace also provides biomarker discovery with AI, especially for immunotherapy applications.