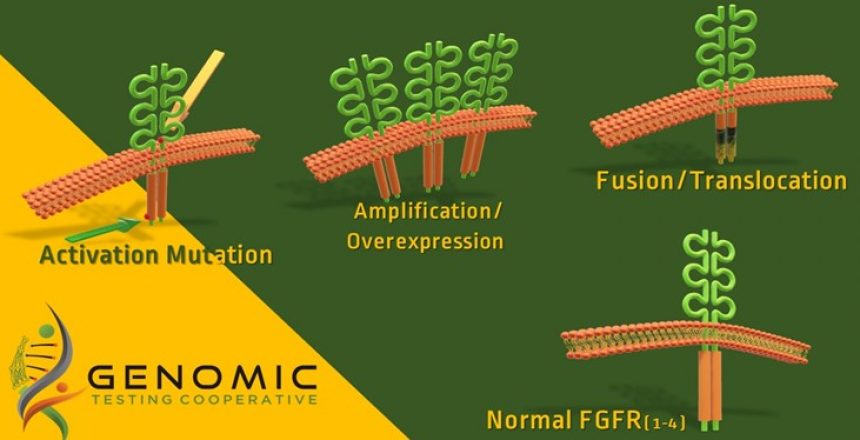
The FDA has approved Balversa (erdafitinib) as a treatment for adult patients with locally advanced or metastatic bladder cancer that have FGFR3 or FGFR2 abnormalities and that has progressed during or following prior platinum-containing chemotherapy.
Urothelial tumors have been reported to show abnormalities in FGFR3 (22%), FGFR1 (8%), and FGFR2 (2%). These abnormalities involve mutations, fusion and amplification/overexpression.
Abnormalities in FGFR can also be seen in various tumor types including lung (20%), breast (10%), melanoma (20%) rhabdomyosarcoma (7%), and cervical cancer (5%).
Genomic Testing Cooperative (GTC) offers comprehensive testing of FGFR(1, 2, 3, and 4) covering mutations in all coding sequence, various fusions and expression levels.
However, mutations studied in the FDA approval process are four point mutations in FGFR3 (FGFR3 gene (p.R248C, p.S249C, p.G370C and p.Y373C) and two fusions (FGFR2:BICC1 and FGFR2:CASP7).
Specimen Requirements:
FFPE: 1 H&E slide and 6-8 unstained slides, 5-7 microns of tissue fixed with 10% NBF fixative. Please circle tumor for microdissection. Alternatively, the FFPE block can be sent for tumor circling and cutting at our laboratory.
Shipping:
Ship using cold pack. The cold pack should not directly contact specimen. Ship as soon as sample collected with overnight delivery.
Turn Around Time: 7 days