Authors: Vanisha Patel1,*, Maciej Kabat1, Andrew Ip2, Sukhdeep Kaur2, Hyung Chan Suh2, Christina Cho2, David Vesole2, Michele Donato2, Maher Albitar2, Scott D. Rowley2
Affiliations:
1Hackensack University Medical Center, Hackensack, New Jersey
2John Theurer Cancer Center, Hackensack, New Jersey
Article history: Received 9 May 2025 | Accepted 11 August 2025
Abstract
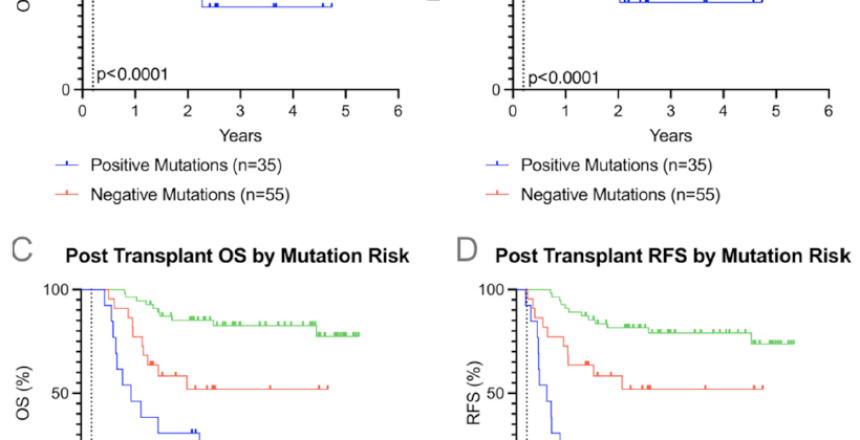
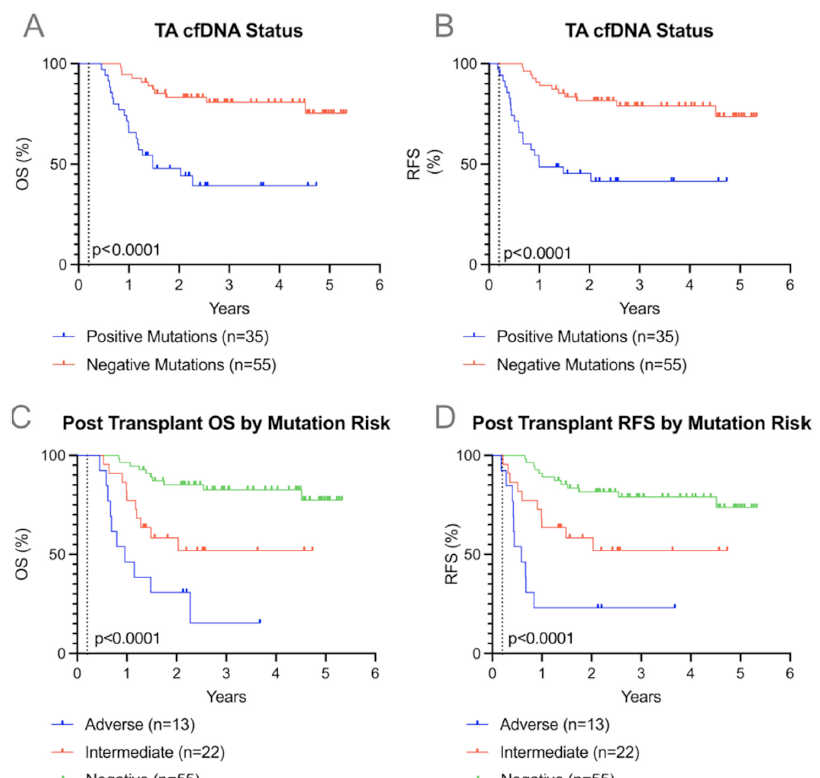
Allogeneic stem cell transplantation (allo-SCT) is a curative option for acute myelogenous leukemia (AML), but relapse is a challenge. Monitoring minimal residual disease post-transplant through detection of tumor-associated circulating cell-free DNA (TA-cfDNA) in peripheral blood (PB) and bone marrow is an emerging strategy to predict relapse. Persistent mutations in TA-cfDNA may be prognostic indicators of relapse and mortality. This single-center retrospective study included 90 AML patients who received allo-SCT from 2018 to 2022, with PB TA-cfDNA tested between Day 100 and 200 after transplantation. TA-cfDNA positivity was determined using commercial genomic sequencing assays reporting tumor-associated genomic alterations with variant allele frequency above 0.01%, and TA-cfDNA negativity was considered the absence of these genomic alterations. The primary endpoints were the association of any TA-cfDNA presence with overall survival (OS) and relapse-free survival (RFS). A secondary endpoint was the association of specific mutation risk (adverse versus intermediate) with OS and RFS. Kaplan–Meier analysis revealed that patients positive for PB TA-cfDNA at Day 150 ± 50 had significantly worse OS (hazard ratio [HR] 5.4, 95% confidence interval [CI] 2.5–11.8, P < .0001) and RFS (HR 5.2, 95% CI 2.4–11.3, P < .0001) compared to TA-cfDNA negative. Regarding mutation risk, adverse mutations at Day 150 ± 50 were linked to worse OS (HR 11.2, 95% CI 3.5–37.2, P < .0001) and RFS (HR 11.6, 95% CI 3.8–36.2, P < .0001) compared to TA-cfDNA negative. This study demonstrates that TA-cfDNA detection in PB post-allo hematopoietic stem cell transplantation is strongly associated with increased relapse and mortality in AML patients. Persistent high-risk mutations correlate with increased risk of relapse and poor survival outcomes. These findings highlight the potential of PB TA-cfDNA as a predictive marker, potentially enabling earlier intervention to alter post-transplant treatment strategies.
© 2025 Published by Elsevier Inc. on behalf of The American Society for Transplantation and Cellular Therapy.
Keywords
Acute myelogenous leukemia; Allogeneic stem cell transplantation; Circulating cell-free DNA; Minimal residual disease; Post-transplant relapse; Molecular monitoring
*Correspondence and reprint requests:
Vanisha Patel, MD, Hackensack University Medical Center, 30 Prospect Avenue, Hackensack, NJ 07601
Email: vanisha.patel@hmhn.org
https://doi.org/10.1016/j.jtct.2025.08.010
Transplantation and Cellular Therapy, Volume 000 (2025), Pages 1–10
Journal homepage: www.astctjournal.org