
Genomics in the Diagnosis, Classification, and Management of Myeloid and Lymphoid Neoplasms
Washington, DCApril 26-27

Washington, DCApril 26-27

John Theurer Cancer presents the 2023 San Antonio Breast Cancer Review: This program, based on data presented at the San Antonio Breast Cancer Symposium 2023, is designed to provide a review of state of the art information on experimental biology, etiology, prevention, diagnosis & therapy of breast cancer/premalignant breast disease to an audience of academic and private physicians and researchers involved in medical, surgical, GYN and radiation therapy, as well as other appropriate health care professionals

This workshop is designed for oncologists, pathologists, oncology nurses and other medical professionals involved in patient care for cancer patients. Saturday, September 23 · 7am – 5pm CDT

Demonstrating Reliability of Targeted Transcriptomic Profiling in Predicting Levels of HER2, ER, PD-L1 and Other Immunohistochemistry-based Biomarkers when Combined with Artificial Intelligence Irvine, California– April

Combining Longitudinal Real-World Data and Clinical Genomics Data Will Enable a More Precise Approach to Cancer Care and Research NEW YORK – COTA, Inc., an
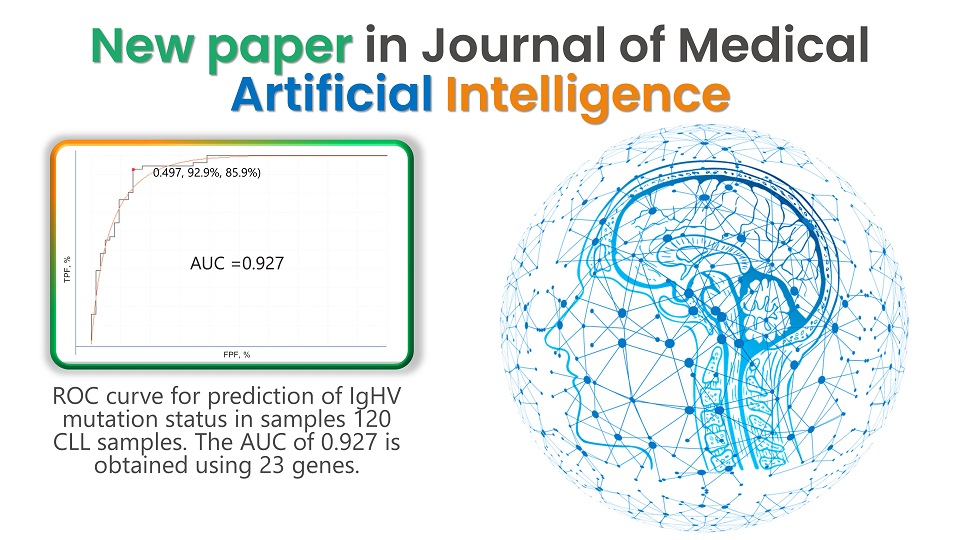
GTC was recently published in the Journal of Medical Artificial Intelligence – Accuracy of predicting IgHV mutation status in chronic lymphocytic leukemia using RNA expression profiling and machine learning.

ASH is just over a month away. Come meet with the GTC team and its co-op partners at our happy hour event. Download your invitation
CHICAGO – With the publication of a paper validating two artificial intelligence-based algorithms for assisting pathologists in molecular profiling of patients, the Genomic Testing Cooperative

SAN FRANCISCO (PRWEB) JUNE 14, 2022. HALO Diagnostics, a precision diagnostics leader, has joined forces with the Genomic Testing Cooperative (GTC) to offer innovative, personalized testing to its physician network and 1M+ patients served. This solution combines HALO Diagnostics’ clinical ensemble and image-guided therapies with GTC’s genomic profiling of a patient’s DNA / RNA using liquid biopsy and tissue samples. Together, the companies will help patients on every step of their unique healthcare journey – from detection and diagnosis to prognosis and treatment.