Myelodysplastic Syndrome (MDS)
MDS is defined as ineffective hematopoiesis characterized by adequate or increased hematologic elements in bone marrow with peripheral cytopenia. Therefore, the presence of cytopenia in

MDS is defined as ineffective hematopoiesis characterized by adequate or increased hematologic elements in bone marrow with peripheral cytopenia. Therefore, the presence of cytopenia in
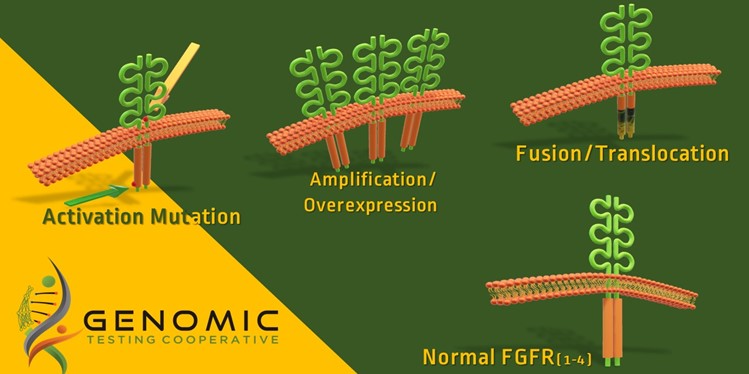
The FDA has approved Balversa (erdafitinib) as a treatment for adult patients with locally advanced or metastatic bladder cancer that have FGFR3 or FGFR2 abnormalities

Genomic Testing Cooperative (GTC) and Collaborators to Present data Resulting from its Proprietary DNA and RNA Profiling of Diffuse Large B-cell Lymphoma at the 2019

Recent study published in Nature Medicine (Rodon et al, Nature Medicine volume 25, pages 751–758, 2019) showed that molecular profiling using both DNA and RNA

A quick interview from Genomic Testing Cooperative founder, CEO/CMO, Dr. Albitar, introducing Genomic Testing Cooperative answering questions about it… Get all the answers by watching
Dr. Albitar’s molecular work is presented at the ASH meeting in oral and poster presentations. Validation of Clinical Prognostic Models and Integration of Genetic Biomarkers
Dr. Albitar’s molecular work is presented at the ASH meeting in oral and poster presentations Program: Oral and Poster Abstracts | Monday, December 3, 2018,
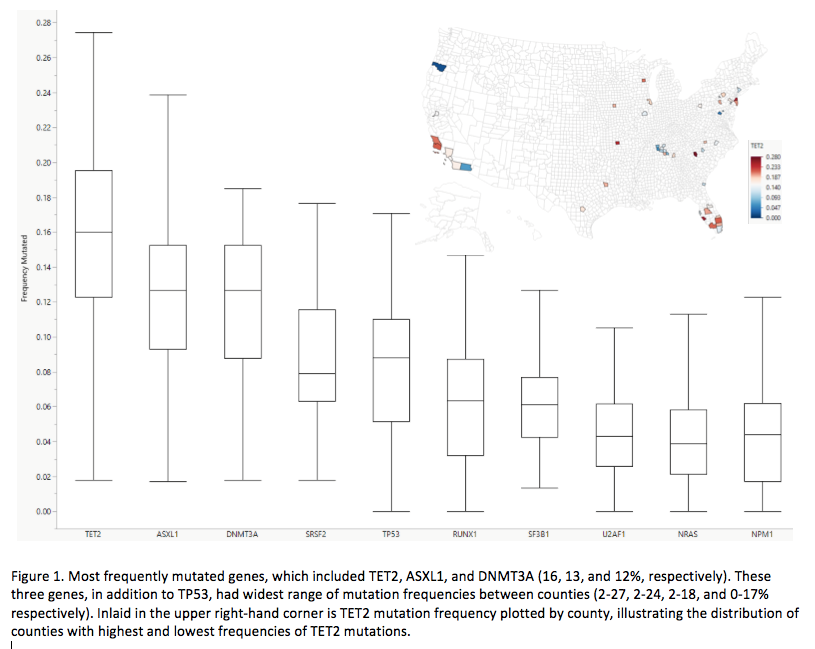
Session: 613. Acute Myeloid Leukemia: Clinical Studies: Poster I Molecular Epidemiologic Associations in Acute Myeloid Leukemia (AML) and Myelodysplastic Syndromes (MDS) within the United States