Genomic Tests
Liquid Biopsy
Solid Tumors
Hematologic Tumors
Patients
Research
Contact Us

ASH 23 Posters
GTC presented abstracts at ASH 2023 GTC’s posters from 2023 Convention of American Society of Hematology are now available to be downloaded. Please reach out
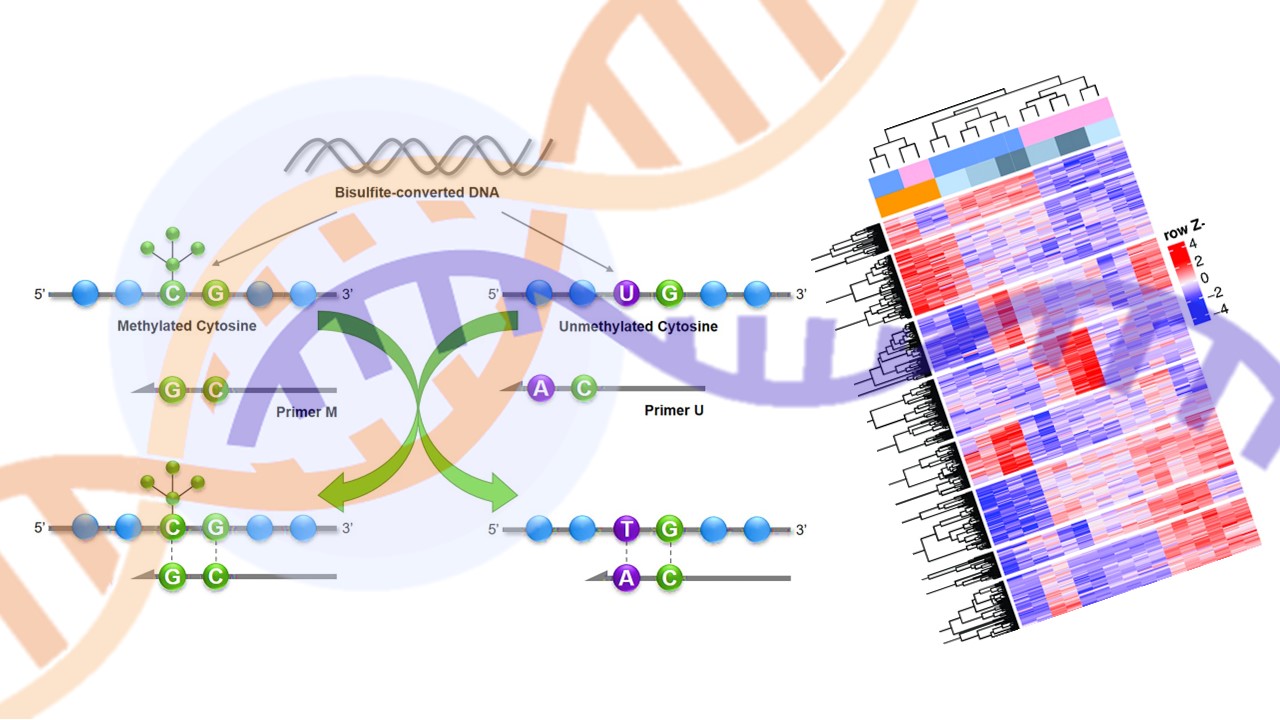
When it comes to early cancer detection and screening which is better? Methylation or Gene Expression
Early cancer detection diagnostic companies have received a lot of attention and venture capital investment over the last few years with numerous labs launching diagnostic tests to address this potentially massive market. While the realized clinical value of these tests for patients is still some way off, many companies have started enrollment in clinical studies and are building databases to advance this technology.
Homologous Recombination Abnormalities Associated With BRCA1/2 Mutations as Predicted by Machine Learning of Targeted Next-Generation Sequencing Data
Homologous recombination deficiency (HRD) is the hallmark of breast cancer gene 1/2 (BRCA1/2)-mutated tumors and the unique biomarker for predicting response to double-strand break (DSB)–inducing drugs. The demonstration of HRD in tumors with mutations in genes other than BRCA1/2 is considered the best biomarker of potential response to these DSB-inducer drugs.
Τumor-informed vs Tumor-naïve
Liquid biopsies are a rapidly evolving diagnostic technology with numerous labs offering commercially available tests today. Most labs have taken an approach of cell-free (cfDNA)





