Chimerism Analysis of Cell-Free DNA in Patients Treated with Hematopoietic Stem Cell Transplantation May Predict Early Relapse in Patients with Hematologic Malignancies.
BACKGROUND:
We studied DNA chimerism in cell-free DNA (cfDNA) in patients treated with HSCT.
METHODS:
Chimerism analysis was performed on CD3+ cells, polymorphonuclear (PMN) cells, and cfDNA using 16 small tandem repeat loci. The resulting labeled PCR-products were size-fractionated and quantified.
RESULTS:
Analyzing samples from 191 patients treated with HSCT for nonneoplastic hematologic disorders demonstrated that the cfDNA chimerism is comparable to that seen in PMN cells. Analyzing leukemia patients (N = 126) showed that, of 84 patients with 100% donor DNA in PMN, 16 (19%) had evidence of clinical relapse and >10% recipient DNA in the plasma. Additional 16 patients of the 84 (19%) showed >10% recipient DNA in plasma, but without evidence of relapse. Eight patients had mixed chimerism in granulocytes, lymphocytes, and plasma, but three of these patients had >10% recipient DNA in plasma compared to PMN cells and these three patients had clinical evidence of relapse. The remaining 34 patients showed 100% donor DNA in both PMN and lymphocytes, but cfDNA showed various levels of chimerism. Of these patients 14 (41%) showed laboratory or clinical evidence of relapse and all had >10% recipient DNA in cfDNA.
CONCLUSION:
Monitoring patients after HSCT using cfDNA might be more reliable than cellular DNA in predicting early relapse.
Reference Image A:
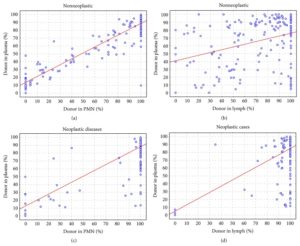
Comparing percent of donor DNA in plasma cfDNA with polymorphonuclear (PMN) and lymphocytes. (a) cfDNA versus PMN DNA in patients with nonneoplastic diseases; (b) cfDNA versus Lymphocytes DNA in patients with nonneoplastic diseases; (c) cfDNA versus PMN in patients with hematologic neoplasms; (d) cfDNA versus lymphocyte DNA in patients with hematologic neoplasms.
Reference Image B:

Chimerism pattern in a patient with CML treated with HSCT. Pretransplant and donor patterns are shown in the left panel. The middle and right panels show the patterns in PMN, lymphocytes, and plasma at two different points. The BCR-ABL fusion level as detected by RT/PCR is shown on top. The middle sample shows mixed chimerism in plasma only as well as residual BCR-ABL1 fusion RNA. The right panel shows the disappearance of the recipient DNA and fusion BCR-ABL1.
Published Online: http://dx.doi.org/10.1155/2016/8589270
References
- E. D. Thomas, R. Storb, R. A. Clift et al., “Bone marrow transplantation,” The New England Journal of Medicine, vol. 292, no. 16, pp. 832–843, 1975. View at Publisher · View at Google Scholar · View at Scopus
- J. O. Armitage, “Bone marrow transplantation,” The New England Journal of Medicine, vol. 330, no. 12, pp. 827–838, 1994. View at Publisher · View at Google Scholar · View at Scopus
- R. Frederick and M. D. Appelbaum, “Hematopoietic-cell transplantation,” The New England Journal of Medicine, vol. 357, pp. 1472–1473, 2007. View at Publisher · View at Google Scholar
- S. Shenoy, T. Mohanakumar, G. Todd et al., “Immune reconstitution following allogeneic peripheral blood stem cell transplants,” Bone Marrow Transplantation, vol. 23, no. 4, pp. 335–346, 1999. View at Publisher · View at Google Scholar · View at Scopus
- N. Novitzky, G. M. Davison, G. Hale, and H. Waldmann, “Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome,” Transplantation, vol. 74, no. 11, pp. 1551–1559, 2002. View at Publisher · View at Google Scholar · View at Scopus
- J. Storek, R. P. Witherspoon, and R. Storb, “T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life,” Bone Marrow Transplantation, vol. 16, no. 3, pp. 413–425, 1995. View at Google Scholar · View at Scopus
- W. Frankel, A. Chan, R. E. T. Corringham, S. Shepherd, A. Rearden, and J. Wang-Rodriguez, “Detection of chimerism and early engraftment after allogeneic peripheral blood stem cell or bone marrow transplantation by short tandem repeats,” American Journal of Hematology, vol. 52, no. 4, pp. 281–287, 1996. View at Publisher · View at Google Scholar · View at Scopus
- A. H. Elmaagacli, D. W. Beelen, H. W. Becks et al., “Molecular studies of chimerism and minimal residual disease after allogeneic peripheral blood progenitor cell or bone marrow transplantation,” Bone Marrow Transplantation, vol. 18, no. 2, pp. 397–403, 1996. View at Google Scholar · View at Scopus
- S.-J. Choi, K.-H. Lee, J.-H. Lee et al., “Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study,” Bone Marrow Transplantation, vol. 26, no. 3, pp. 327–332, 2000. View at Publisher · View at Google Scholar · View at Scopus
- J. Serrano, J. Roman, J. Sanchez et al., “Molecular analysis of lineage-specific chimerism and minimal residual disease by RT-PCR of p210BCR-ABL and p190BCR-ABL after allogeneic bone marrow transplantation for chronic myeloid leukemia: increasing mixed myeloid chimerism and p190BCR-ABLdetection precede cytogenetic relapse,” Blood, vol. 95, no. 8, pp. 2659–2665, 2000. View at Google Scholar· View at Scopus
- A. H. Elmaagacli, K. Runkel, N. Steckel et al., “A comparison of chimerism and minimal residual disease between four different allogeneic transplantation methods in patients with chronic myelogenous leukemia in first chronic phase,” Bone Marrow Transplantation, vol. 27, no. 8, pp. 809–815, 2001. View at Publisher · View at Google Scholar · View at Scopus
- M. Lawler, P. Humphries, and S. R. McCann, “Evaluation of mixed chimerism by in vitro amplification of dinucleotide repeat sequences using the polymerase chain reaction,” Blood, vol. 77, no. 11, pp. 2504–2514, 1991. View at Google Scholar · View at Scopus
- I. Buño, P. Nava, A. Simón et al., “A comparison of fluorescent in situ hybridization and multiplex short tandem repeat polymerase chain reaction for quantifying chimerism after stem cell transplantation,” Haematologica, vol. 90, no. 10, pp. 1373–1379, 2005. View at Google Scholar · View at Scopus
- M. Adamek, G. Opelz, K. Klein, C. Morath, and T. H. Tran, “A fast and simple method for detecting and quantifying donor-derived cell-free DNA in sera of solid organ transplant recipients as a biomarker for graft function,” Clinical Chemistry and Laboratory Medicine, vol. 53, pp. 1434–6621, 2015. View at Publisher · View at Google Scholar
- J. Beck, M. Oellerich, U. Schulz et al., “Donor-derived cell-free DNA is a novel universal biomarker for allograft rejection in solid organ transplantation,” Transplantation Proceedings, vol. 47, no. 8, pp. 2400–2403, 2015. View at Publisher · View at Google Scholar
- R. Sachidanandam, D. Weissman, S. C. Schmidt et al., “A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms,” Nature, vol. 409, no. 6822, pp. 928–933, 2001.View at Publisher · View at Google Scholar · View at Scopus
- J. R. Gomez, J. Serrano, A. Jiménez et al., “Myeloid mixed chimerism is associated with relapse in bcr-abl positive patients after unmanipulated allogeneic bone marrow transplantation for chronic myelogenous leukemia,” Haematologica, vol. 85, no. 2, pp. 173–180, 2000. View at Google Scholar · View at Scopus
- P. Bader, J. Beck, A. Frey et al., “Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT,” Bone Marrow Transplantation, vol. 21, no. 5, pp. 487–495, 1998. View at Publisher · View at Google Scholar ·View at Scopus


