
ASCO 24 Posters
GTC presented abstracts at ASCO 2024 GTC’s posters from 2024 Convention of American Society of Clinical Oncology are now available to be downloaded. Please reach

GTC presented abstracts at ASCO 2024 GTC’s posters from 2024 Convention of American Society of Clinical Oncology are now available to be downloaded. Please reach

GTC presented abstracts at ASH 2023 GTC’s posters from 2023 Convention of American Society of Hematology are now available to be downloaded. Please reach out
Liquid biopsies are a rapidly evolving diagnostic technology with numerous labs offering commercially available tests today. Most labs have taken an approach of cell-free (cfDNA)

GTC presented abstracts at ASCO 2023 GTC’s posters from 2023 Convention of American Society of Clinical Oncology are now available to be downloaded. Please reach
GTC presented abstracts at AACR 2023 GTC’s posters from 2023 Convention of American Association for Cancer Research are now available to be downloaded. Please reach

Baltimore, MD and Irvine, CA, April 13, 2023, Sysmex Inostics Inc., a subsidiary of Japan’s Sysmex Corporation and Baltimore-based biotechnology firm, and Genomic Testing Cooperative

Combining Longitudinal Real-World Data and Clinical Genomics Data Will Enable a More Precise Approach to Cancer Care and Research NEW YORK – COTA, Inc., an
CHICAGO – With the publication of a paper validating two artificial intelligence-based algorithms for assisting pathologists in molecular profiling of patients, the Genomic Testing Cooperative
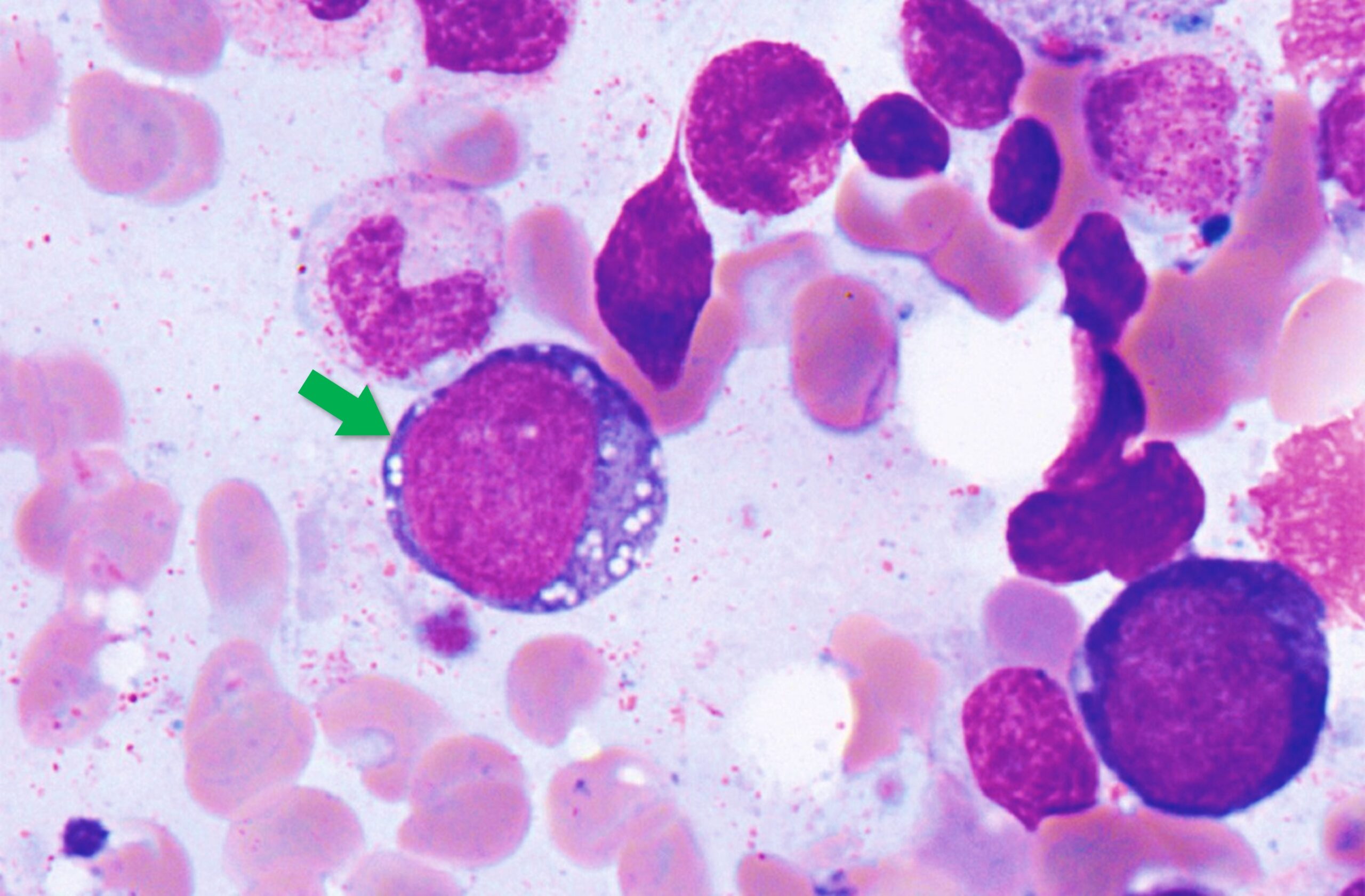
Background VEXAS syndrome is a disease with inflammatory and hematologic (blood) manifestations. The syndrome is caused by mutations in the UBA1 gene affecting the Met41 residue of
Irvine, California– June 1, 2022 – Genomic Testing Cooperative, LCA (GTC) announced that it will be presenting at the American Society of Clinical Oncology (ASCO)