Overview
Liquid Trace™ on cerebrospinal fluid (CSF) is a comprehensive liquid biopsy specifically optimized for primary and metastatic central nervous system (CNS) neoplasms. The assay detects tumor-derived cfDNA and cfRNA and other molecular signals in CSF to support diagnosis, genomic profiling, measurable residual disease (MRD) monitoring, selecting therapy, and clinical trial matching.
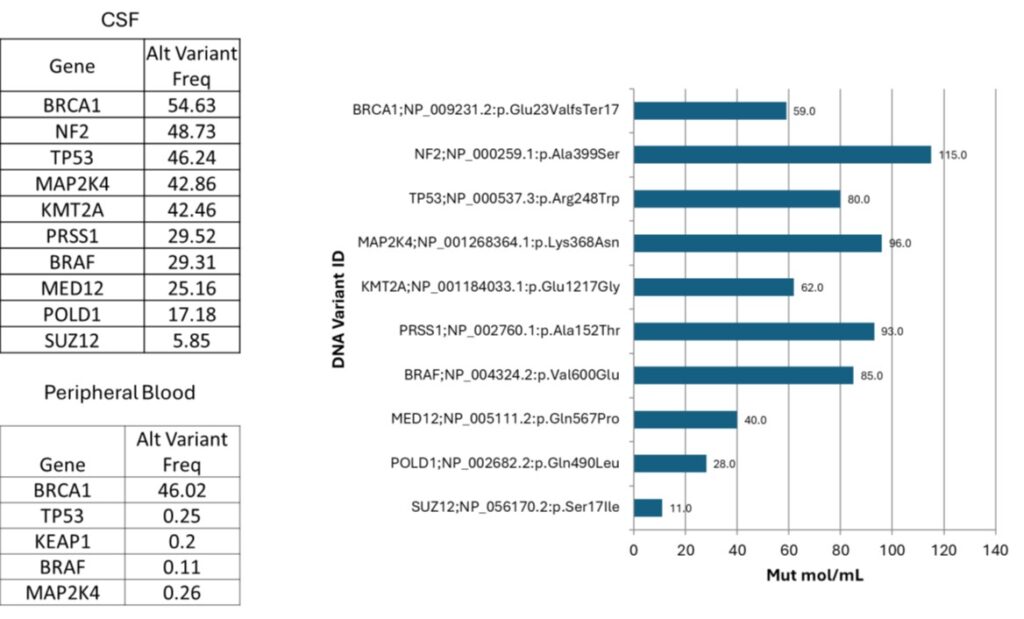
Key features
- Sensitive detection of tumor-derived genomic material in CSF (higher yield than plasma for CNS-limited disease).
- Broad analyte panel: single-nucleotide variants, indels, gene fusions, copy number changes (amplification/deletion), gene expression, HLA genotyping and chromosomal alterations.
- Quantification capabilities: tumor mutational burden (TMB) and measurable residual disease (MRD) by mutant molecules per 1mL CSF.
- Lymphoid clonality testing (T-cell and B-cell) to aid diagnosis of CNS lymphoma and leukemia
- Viral detection of HPV, EBV, TTV, HTLV-1
- Optional add-on testing: MGMT promoter methylation analysis for brain tumors when tumor DNA is detected.

Clinical indications and utility
- Primary brain tumors: Detects alterations in high-grade gliomas (e.g., glioblastoma) and other primary CNS neoplasms; supports diagnosis and informs targeted therapy selection.
- Leptomeningeal and parenchymal brain metastases: Identifies intracranial driver mutations (EGFR, ALK, etc.) that may guide CNS-penetrant targeted therapies.
- CNS lymphoma/leukemia: Combines mutation detection (e.g., MYD88) with T- and B-cell clonality to improve diagnostic sensitivity.
- Patients unable to undergo biopsy: CSF provides a minimally invasive alternative to obtain actionable genomic information.

Why CSF over plasma
CSF is enriched for tumor-derived nucleic acids in CNS-limited disease, often yielding higher sensitivity and a more accurate picture of intracranial tumor genetics than plasma. Testing CSF can reveal alterations absent from contemporaneous tissue biopsies because it samples a broader representation of intracranial tumor heterogeneity.

What the report includes
- Full list of actionable somatic variants and copy number changes relevant to targeted therapy selection and clinical trials (examples: NTRK1/2/3 fusions; CDKN2A/B deletions; amplifications such as EGFR, MYC/MYCN).
- Recommended interpretations for therapy and trial matching.
- Quantitative metrics: TMB and MRD estimates when applicable.
- Clonality analysis for lymphoid neoplasms and viral RNA detection where relevant.

Ordering, specimen requirements & practical notes
- Specimen: CSF collected via lumbar puncture or Omaya reservoir. Ship per instructions provided with GTC kits.
- Add-ons: Request MGMT methylation analysis or HLA genotyping as required; consider testing both tissue and CSF to maximize sensitivity. “Don’t accept partial results” — missing complementary data can impact clinical decisions.
- Turnaround & support: Reports include interpreted results and treatment/trial-matching guidance. Clinical-laboratory consultation is available for result review.

How to learn more or request testing
Contact Genomic Testing Cooperative to request CSF collection kits, discuss test selection, or arrange a case consultation:
Genomic Testing Cooperative | Toll-Free: 1-866-484-8870 | Tel: 1-949-540-9421. Full assay details and sample instructions are available from GTC’s laboratory team.
