Press Releases
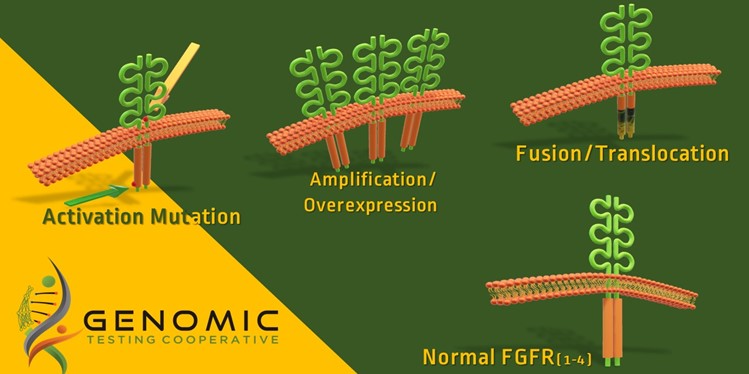
The FDA has Approved Drugs for FGFR Abnormalities
The FDA has approved Balversa (erdafitinib) as a treatment for adult patients with locally advanced or metastatic bladder cancer that have FGFR3 or FGFR2 abnormalities

The FDA has approved Balversa (erdafitinib) as a treatment for adult patients with locally advanced or metastatic bladder cancer that have FGFR3 or FGFR2 abnormalities

Genomic Testing Cooperative (GTC) and Collaborators to Present data Resulting from its Proprietary DNA and RNA Profiling of Diffuse Large B-cell Lymphoma at the 2019

Recent study published in Nature Medicine (Rodon et al, Nature Medicine volume 25, pages 751–758, 2019) showed that molecular profiling using both DNA and RNA