People over the age of 50 are at increased risk for hematologic and cardiovascular diseases. Testing for clonal hematopoiesis helps in evaluating such risks.
This is a next-generation sequencing (NGS) test to identify molecular abnormalities in 65 genes implicated in hematologic cells. The assay is designed to detect genomic abnormalities in hematologic cells. The presence of genomic abnormalities indicates clonal hematopoiesis. Clonal hematopoiesis has implication on the development of hematologic neoplasms as well as cardiovascular diseases.
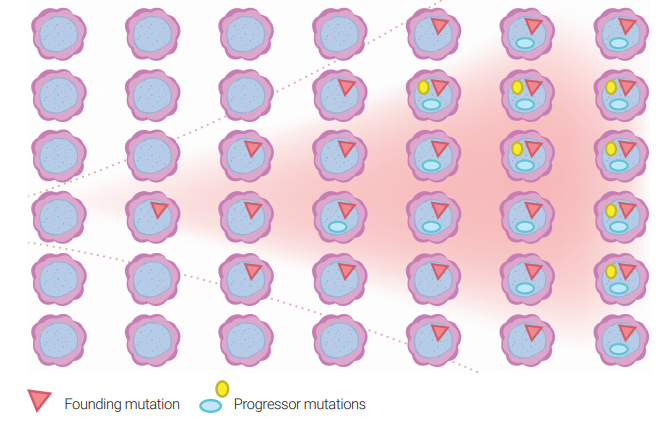
Clonal hematopoiesis of indeterminate potential (CHIP) is defined by the presence of low-level mutations in the peripheral blood in clinically normal individuals. The rate of transformation to a hematological neoplasia is 0.5-1% per year. Clonal cytopenias of undetermined significance (CCUS) is defined by the presence of cytopenia (anemia, low platelets or white cells) along with llow-level mutations.
CHIP is detected in 3-5% of normal individuals above the age of 50 and in approximately 10% of people aged 70 to 80.
The diagnosis of MDS is confirmed when mutations in hematopoietic cells are detected at relatively high levels (>40% of cells). However, mutations at low levels in a few cells can be detected in normal individuals.
The incidence of MDS is 3-4 per 100,000 per year but
increases significantly above the age of 50 and it is at
30 per 100,000 per year in patients above the age of 70.
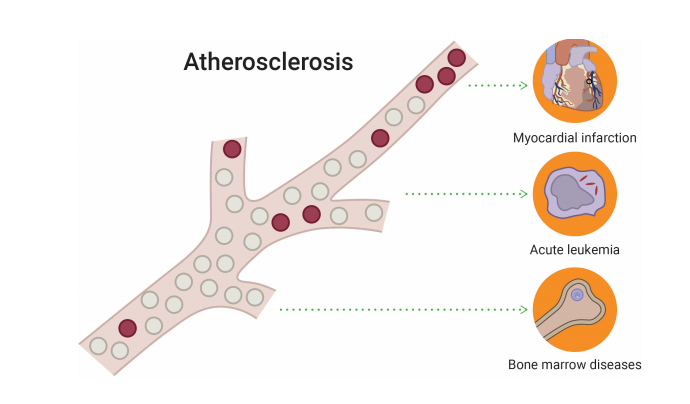
Patients with CHIP have 4.0 times greater risk of myocardial infarction as compared to individuals without such clone. The prevalence of CHIP in patients with coronary artery disease is reported to be at 18.2%. Mutated genes in CHIP, are pro-inflammatory and lead to the development of atherosclerotic plaques. Anti-inflammatory agents might slow the progression of cardiovascular disease in patients with low-level mutations in peripheral blood.
Approximately 25% to 65% of patients
with cytopenia will have mutation in one or more genes.
These patients with mutations have a significantly higher probability of developing MDS or other hematopoietic neoplasms (AML, MPN, lymphoma, etc.) within 5 years.
Testing the peripheral blood for the presence of mutations provides information for:
- Potential early diagnosis of myelodysplastic syndrome (MDS)
- Detecting the presence of mutations in the presence of cytopenia and confirming the diagnosis of clonal cytopenias of undetermined significance (CCUS)
- Detecting CHIP and predicting an increased risk of cardiovascular disease (CVD)
- Detecting the presence of CHIP to monitor the development of hematologic neoplasms, especially MDS
Specimen Requirements: 5mL peripheral blood, EDTA tube
How to complete the Genomic Testing Cooperative requisition form.
Download our
Test Requisition
Keep in mind that we do not accept blood samples directly from individuals. Talk with your M.D. to fill out the form for you.
